Please select one:
EYES ON A BRIGHTER FUTURE: EMERGING IRD TREATMENTS
Ramya, living with an IRD

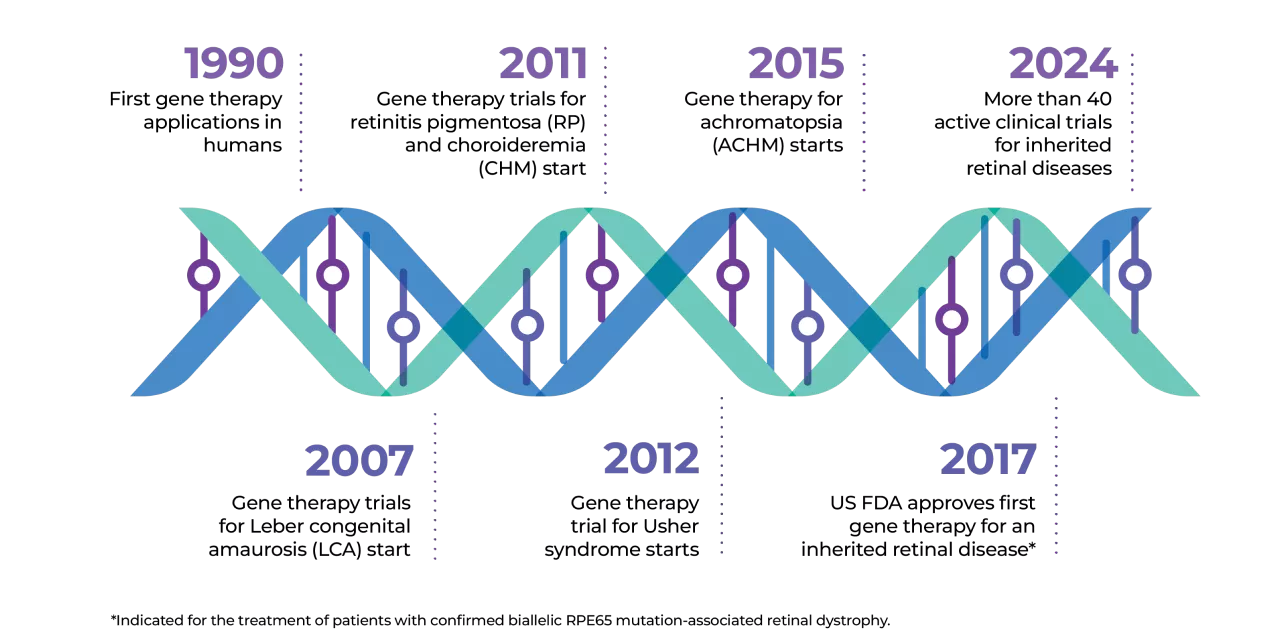
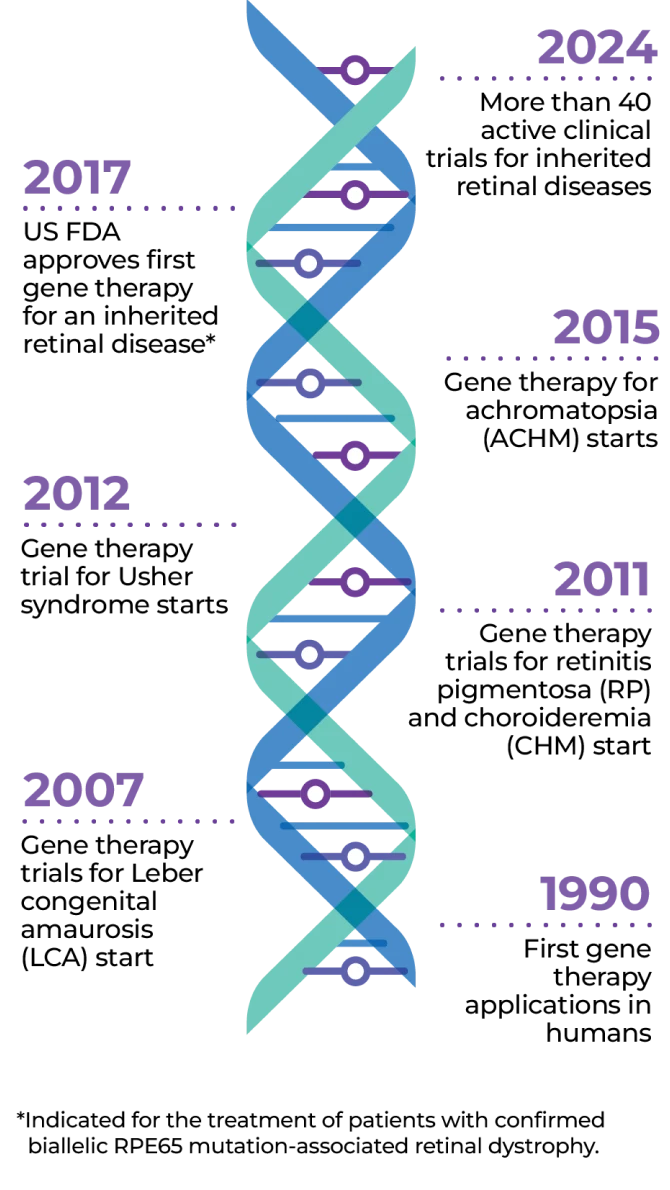
Gene therapy is an exciting treatment advance that changes a specific part of a person’s genes to treat disease. The first gene therapy for an inherited retinal disease (IRD) was approved by the FDA in 2017.* Today there are more than 40 ongoing clinical trials to find a solution for people living with IRD.
After receiving your results, your genetic counselor and eye specialist may recommend extra steps to find out if you are eligible for a clinical trial or for approved or emerging therapies.
Learn more about ongoing trials hereTypes of gene therapy
Many gene therapy innovations are now being studied. Each therapy (below) is designed to affect genes associated with inherited retinal disease in a special way.
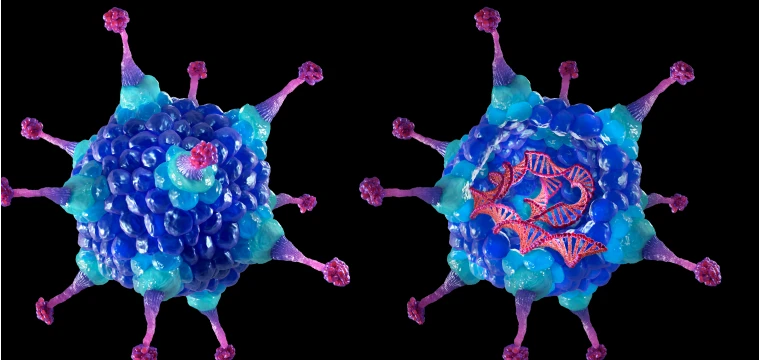
Gene replacement therapy
Gene replacement therapy, also known as augmentation therapy, works by inserting normal copies of the mutated gene into the host cells
Gene editing
Gene editing corrects the gene variant directly within the host DNA

RNA editing
RNA (ribonucleic acid) editing edits the RNA, not the DNA
The eye is an ideal focus for gene therapy.
Here’s why:
- Treatment can be delivered directly to the eye with a one-time outpatient procedure
- Results are easy to observe, making it a relatively easier method for an eye specialist to track your progress
- Any side effects that might happen tend to be localized to the eye and systemic side effects are limited
It is important to share the facts on gene therapy

More than 40 clinical trials have been completed or are underway for different types of IRDs. Gene therapy exists for many other genetic conditions as well.

There are already US FDA- and EMA-approved gene therapies on the market, including one for IRDs.

There are gene therapies that only change DNA in the cells of the body, called somatic cells, which are not passed along to your children.